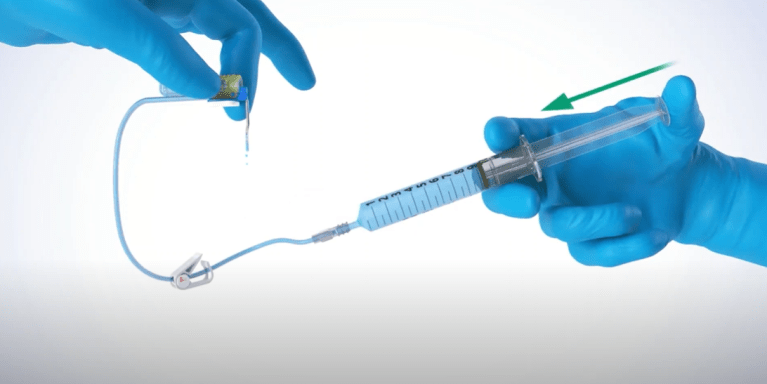
Safety Huber needle
PPS CT
Description
The PPS® CT is a curved safety Huber needle supplied with one connecting line ideal for accessing pressure injectable ports. This device can be… Read more
Indication
PPS® CT safety Huber needles are indicated for:
•• Administration or withdrawal of fluids through implantable ports.
•• Pressure injection of contrast media into the central venous system only with an implantable infusion port that is also indicated for pressure injections.
The maximum recommended infusion rate at 11.8 cPs is:
–– 5 mL/sec for a 19G needle. (1.1 mm)
–– 5 mL/sec for a 20G needle. (0.9 mm)
–– 2 mL/sec for a 22G needle. (0.7 mm)
PPS® CT safety Huber needles are indicated to prevent blood-borne pathogen exposures caused by accidental needle punctures (accidental blood exposure). They do not protect against other routes of blood-borne pathogen transmission.
Product highlights
Description
The PPS® CT is a curved safety Huber needle supplied with one connecting line ideal for accessing pressure injectable ports. This device can be used for pressure injection of contrast media (up to 300 psi / 2068 kPa maximum) during CT scan (Computerized Tomography) or MRI (Magnetic Resonance Imaging) exams, and is MR Conditional (see details in parts V of this IFU).The PPS® CT curved safety Huber needle is available:
•• in several lengths and diameters
•• with or without Y-site
PPS® CT codes 80xxxx are without a Y site.
PPS® CT codes 81xxxx are with a Y site.
It is supplied with:
•• Luer-Lock connectors on which closed protective caps are screwed (end of the main line and of the Y site) which must not be used as sealing caps.
•• and one clamp (2 clamps for references including an Y-site) indicating the maximum flow rate of the PPS® CT.
The PPS® CT is not composed of natural latex (raw-materials and packaging) with DEHP-free tubing.
References and Features
| Needle | Packaging | ||||||
|---|---|---|---|---|---|---|---|
| Favourites | Code | Diameter G | Ext. Ø mm | Length mm | Priming volume ml | Units/Box | Units/Case |
| 801507 | 22 | 0.7 | 15 | 0.50 | 12 | 360 | |
| 801509 | 20 | 0.9 | 15 | 0.50 | 12 | 360 | |
| 801511 | 19 | 1.1 | 15 | 0.50 | 12 | 360 | |
| 801707 | 22 | 0.7 | 17 | 0.50 | 12 | 360 | |
| 801709 | 20 | 0.9 | 17 | 0.50 | 12 | 360 | |
| 801711 | 19 | 1.1 | 17 | 0.50 | 12 | 360 | |
| 802007 | 22 | 0.7 | 20 | 0.50 | 12 | 360 | |
| 802009 | 20 | 0.9 | 20 | 0.50 | 12 | 360 | |
| 802011 | 19 | 1.1 | 20 | 0.50 | 12 | 360 | |
| 802507 | 22 | 0.7 | 25 | 0.50 | 12 | 360 | |
| 802509 | 20 | 0.9 | 25 | 0.50 | 12 | 360 | |
| 802511 | 19 | 1.1 | 25 | 0.50 | 12 | 360 | |
| 803007 | 22 | 0.7 | 30 | 0.50 | 12 | 360 | |
| 803009 | 20 | 0.9 | 30 | 0.50 | 12 | 360 | |
| 803011 | 19 | 1.1 | 30 | 0.50 | 12 | 360 | |
| 803507 | 22 | 0.7 | 35 | 0.50 | 12 | 360 | |
| 803509 | 20 | 0.9 | 35 | 0.50 | 12 | 360 | |
| 803511 | 19 | 1.1 | 35 | 0.50 | 12 | 360 | |
| 811507 | 22 | 0.7 | 15 | 0.70 | 12 | 360 | |
| 811509 | 20 | 0.9 | 15 | 0.70 | 12 | 360 | |
| 811511 | 19 | 1.1 | 15 | 0.70 | 12 | 360 | |
| 811707 | 22 | 0.7 | 17 | 0.70 | 12 | 360 | |
| 811709 | 20 | 0.9 | 17 | 0.70 | 12 | 360 | |
| 811711 | 19 | 1.1 | 17 | 0.70 | 12 | 360 | |
| 812007 | 22 | 0.7 | 20 | 0.70 | 12 | 360 | |
| 812009 | 20 | 0.9 | 20 | 0.70 | 12 | 360 | |
| 812011 | 19 | 1.1 | 20 | 0.70 | 12 | 360 | |
| 812507 | 22 | 0.7 | 25 | 0.70 | 12 | 360 | |
| 812509 | 20 | 0.9 | 25 | 0.70 | 12 | 360 | |
| 812511 | 19 | 1.1 | 25 | 0.70 | 12 | 360 | |
| 813007 | 22 | 0.7 | 30 | 0.70 | 12 | 360 | |
| 813009 | 20 | 0.9 | 30 | 0.70 | 12 | 360 | |
| 813011 | 19 | 1.1 | 30 | 0.70 | 12 | 360 | |
| 813507 | 22 | 0.7 | 35 | 0.70 | 12 | 360 | |
| 813509 | 20 | 0.9 | 35 | 0.70 | 12 | 360 | |
| 813511 | 19 | 1.1 | 35 | 0.70 | 12 | 360 | |
Additional information
-
Contains Latex No
-
Contains animal product No
-
Pyrogen-free No
Documentation
-
Brochure_PPS_CT
-
Brochure Vascular Access Range
-
Instructions for use